Before engaging in work on the isolation and analyses of extracellular vesicles (EVs), I honestly was not even aware that EVs could play such diverse roles in the pathophysiology of disease. In tumors, for example, EVs can regulate tumor immune responses, initiate formation of a pre-metastatic niche, determine organotropic metastasis, and contribute to chemotherapeutic resistance1,2 These new findings keep changing our understanding of EVs. In contrast, the undergraduate textbook that I used simply introduced EVs as garbage bags used to discard obsolete cytosolic and molecular remnants3. However, to further explore the biological roles of EVs and potentially translate them into clinical applications, it is important to have better techniques to isolate EVs for subsequent study. The existing approaches, which are protein-, size-, density- or solubility-dependent4, generally require at least a few hours to finish the isolation procedure. For this reason, a scalable platform without bulky equipment or complex pre-treatment for rapid and efficient isolation of EVs is highly desirable.
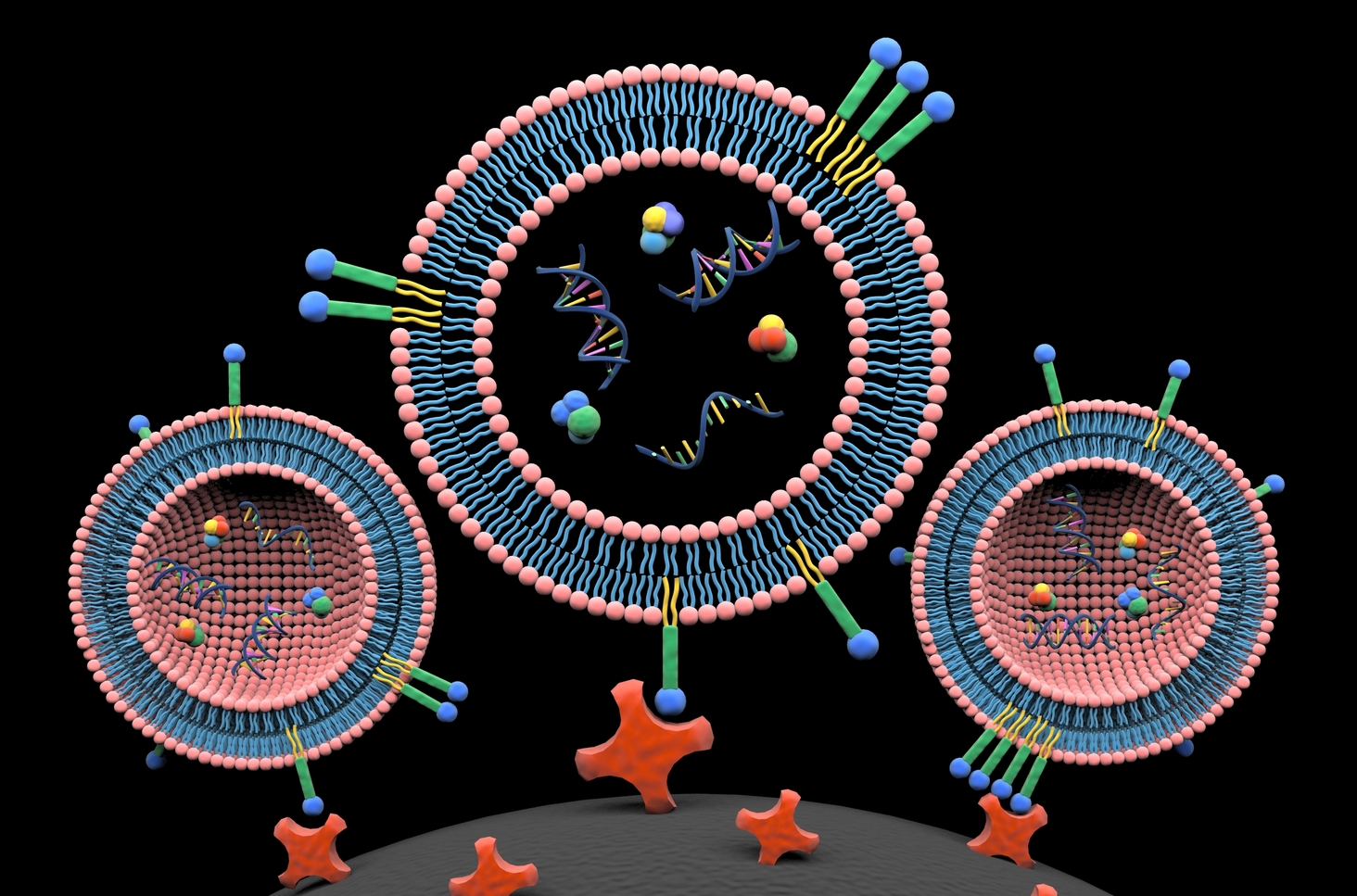
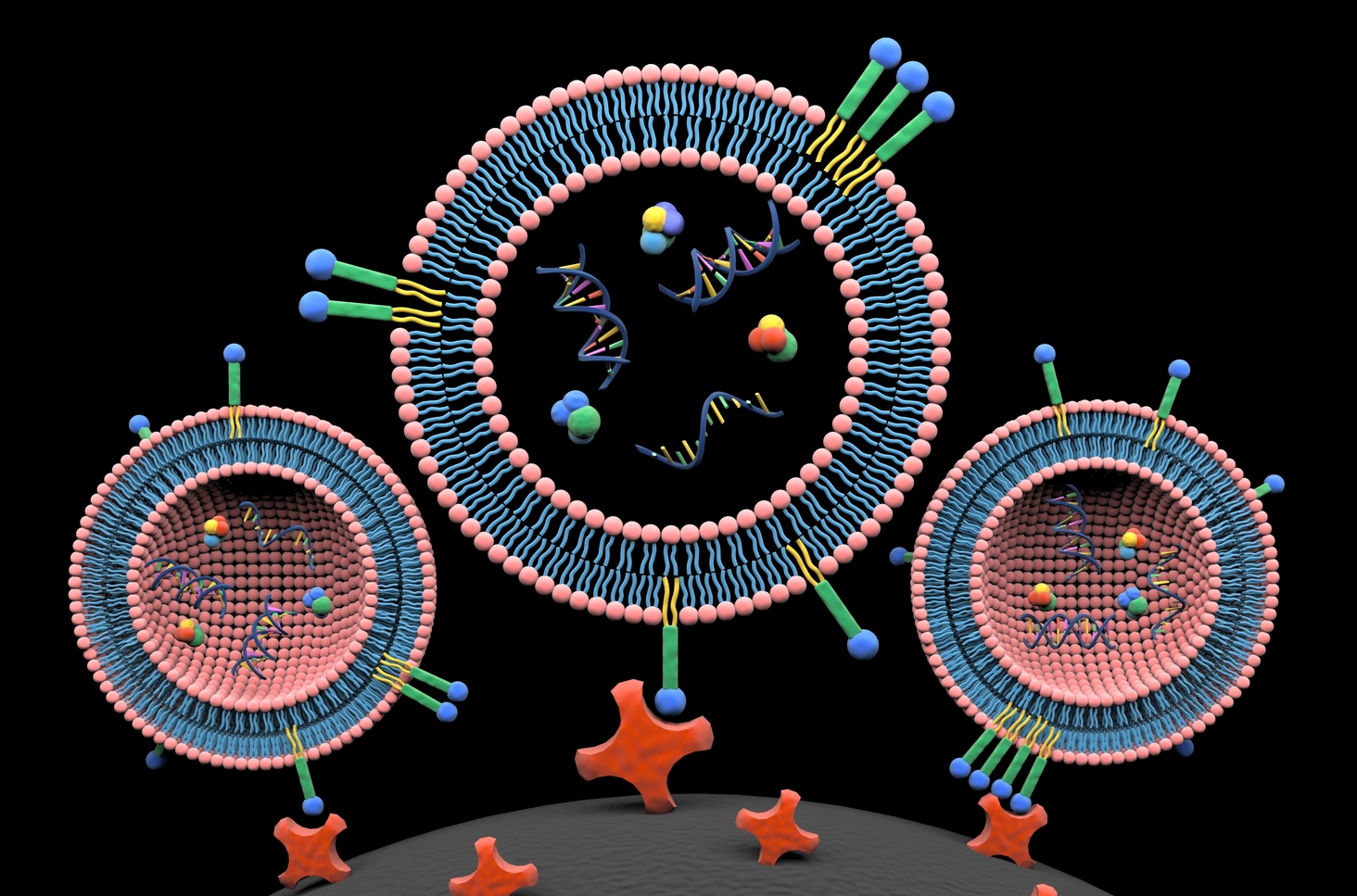
Our first attempts to isolate EVs utilized methods that fell into existing categories. But after months of pursuing traditional paths, we failed to make a real breakthrough. At a loss as to how to proceed, I struck up a conversation with Dr. Siyang Zheng in which I mentioned the possibility of labeling the lipid envelope that encloses EVs, a structure that proteins do not have. Dr. Zheng took up this thought, and discussed how dye–ligand affinity chromatography had been developed to isolate proteins5. Inspired by this, we turned our focus to dyes, found that there were many membrane probes6, and learned that lipids had been widely used for cell manipulation and drug delivery, due to their low toxicity and stable retention7,8. These materials are pretty standard for biomedical researchers who are working on cell biology and pharmacy. However, lipids are infrequently used in molecular separation and have never been used for enrichment of EVs. Three commercially available lipid probes (monoacyl lipid, diacyl lipid, and cholesterol) were finally selected for isolation of EVs. I would note that the ideal lipid probe should offer maximal labeling efficiency, while minimizing non-specific protein binding. Although we used a diacyl lipid, called DSPE, for this study, we would like to further engineer and optimize such lipid nanoprobes for EV isolation.
As novices without any experience in EV isolation and analyses, we took a zigzag course, learned from the exploration, and gained much experience from failures. For example, we found that both proteins and EVs can readily absorb to surfaces of common polystyrene tubes9, resulting in much less output of EVs. In mutation detection of EV-derived DNA, we fortunately identified one mutation in a patient sample using Sanger sequencing on the first attempt. Then, we realized that a mutation detection assay with significantly high sensitivity and specificity probably needed to be routine10,11. In mass spectrometer analyses of EV cargo proteins, after many failures we were aware of the fact that gel slicing into pieces for protein purification and recovery are absolutely necessary12. There were many other experimental minutia that also must be carefully approached. In accomplishing this work we benefitted from solving numerous small problems. Aided with the approach that we developed, the isolation procedure of EVs can be decreased to 15 minutes, and the isolation efficiency and cargo composition is similar to what can be achieved by ultracentrifugation. Moreover, our approach demonstrates less protein contaminants and would facilitate proteomic profiling of EVs. In clinical translation, this approach potentially can promote EV-based liquid biopsy for cancer diagnosis, prognosis, and treatment monitoring.
In retrospect, we believe that a comprehensive literature review and a well-organized research scheme are of primary importance, though we do not exclude the chance of unexpected discoveries. This study shows that the introduction of tools and concepts from other disciplines can provide a fresh direction to one’s research. Finally, the benefits of enthusiasm and curiosity in research and study can hardly be overstated.
Our paper: Wan, Y. et al. Rapid magnetic isolation of extracellular vesicles via lipid-based nanoprobes. Nat. Biomed. Eng. 1, 0058 (2017).
References:
1. Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
2. Andaloussi, S.E., Mäger, I., Breakefield, X.O. & Wood, M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013).
3. Johnstone, R. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem. Cell Biol. 70, 179–190 (1992).
4. Liga, A., Vliegenthart, A., Oosthuyzen, W., Dear, J. & Kersaudy-Kerhoas, M. Exosome isolation: a microfluidic road-map. Lab. Chip 15, 2388–2394 (2015).
5. Denizli, A. & Pişkin, E. Dye-ligand affinity systems. J. Biochem. Biophys. Meth. 49, 391–416 (2001).
6. GUI, A. Molecular Probes™ Handbook.
7. Mueller, R.H., Maeder, K. & Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 50, 161–177 (2000).
8. Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
9. Goebel-Stengel, M., Stengel, A., Taché, Y. & Reeve, J.R. The importance of using the optimal plasticware and glassware in studies involving peptides. Anal. Biochem. 414, 38–46 (2011).
10. Bettegowda, C. et al. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci. Trans. Med. 6, 224ra224 (2014).
11. Newman, A.M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 20, 548–554 (2014).
12. Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 113, E968–E977 (2016).



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in