Imaging of small animals, especially rodents, provides physiological, pathological, and phenotypical insights into the most relevant milieu—an intact, living system—and has played an indispensable role in preclinical research. In past decades, preclinical small-animal wholebody imaging has typically relied on non-optical imaging modalities, including magnetic resonance imaging (MRI), X-ray computed tomography (X-ray CT), positron emission tomography (PET) or single-photon emission computed tomography (SPECT), and ultrasound tomography (UST). These modalities all have excellent imaging depth, seeing through the whole animal. However, their intrinsic limitations prevent them from being complete solutions for comprehensive preclinical studies. For example, MRI requires a long data acquisition time, ranging from seconds to minutes, too slow for imaging dynamics, especially for imaging fast neural activities.1 X-ray CT and PET/SPECT use ionizing radiation, which can inhibit longitudinal monitoring of tumor growth or drug regimens.2 UST lacks extravascular molecular contrasts, and so cannot image molecular pathways and tissue metabolism.3
Photoacoustic tomography (PAT) is a hybrid imaging modality that acoustically detects optical absorption contrast via the photoacoustic effect, in which absorbed optical energy is converted into acoustic energy. By combining optical excitation with acoustic detection, PAT realizes both desirable optical contrasts and superior acoustic imaging depth. PAT holds great promise as a full-package solution for small-animal wholebody imaging. However, the performance and image quality of established PAT systems fall short, providing either limited view artifacts or blurry structural images with low contrast. After years of technical refinement, we decided to undertake the grand engineering challenge of achieving high contrast imaging of whole-body details inside rodents.
When designing this system, we optimized almost every aspect of the technology, including the laser illumination, acoustic detection, signal transmission and digitization, image reconstruction, and post processing. To avoid artifacts produced by non-uniform illumination via fiber delivery, we designed a side illumination path for free-space light delivery, which enabled practically unlimited delivered pulse energy and uniform illumination. The structures and features inside the organs span all angular directions, so we need a panoramic view to fully cover them. Hence, we employed a full-ring transducer array to give full view detection coverage. To deliver whole-body level imaging, we needed a field of view (FOV) large enough to encompass the whole cross-section of a rodent’s trunk. According to the spatial Nyquist sampling criterion, the FOV is directly related to the number of elements in the array, but this relationship has never been thoroughly investigated before in the field of PAT. Carefully working through the math, we found that 512 elements would provide the desired performance. As another optimization, we realized that most array-based PAT systems amplify the received photoacoustic signal after it has traveled through a long cable, which introduces noise and contaminates the true signal. To amplify the true signal before contamination, we engineered a set of pre-amplifiers, each directly connected to an ultrasonic transducer element. We employed four commercial data acquisition units, each of which supported 128 channels, and developed a control program to synchronize the four units for 512-channel parallel digitization. At present, we can acquire a two-dimensional cross-sectional image with a single laser shot, and the overall data acquisition time is less than 50 µs, which is fast enough to observe most biological processes above the cellular scale with no motion artifacts. We found that images reconstructed using conventional algorithms displayed artifacts due to acoustic heterogeneities, including horseshoe-shaped features on the body surface and splitting of the vasculature inside organs. Thus we developed a dual-speed-of-sound universal back-projection algorithm to address the acoustic heterogeneities and completely remove such artifacts. The synergistic combination of all those individual innovations has yielded, in our opinion, the highest-quality small animal whole-body images ever achieved by our field.

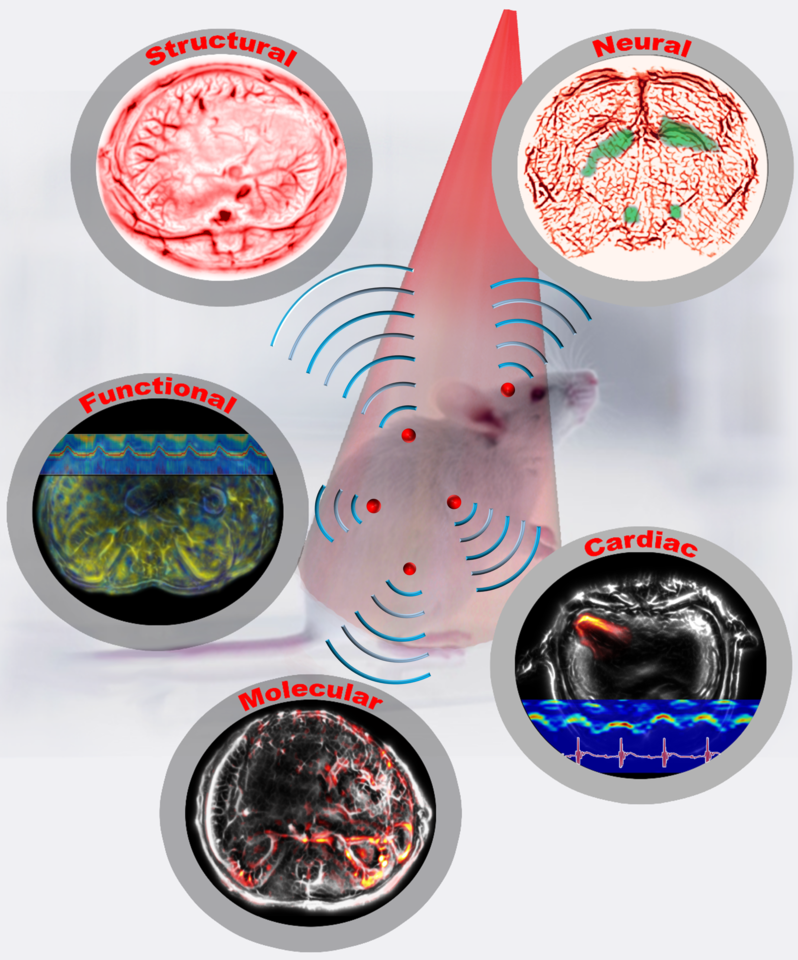
Figure 1: Single-flash multi-contrast imaging of rodents
Based on whole-body movies of detailed sub-organ structures inside a breathing mouse and imaging through a rat’s whole brain, we confidently envision widespread applications in preclinical drug screening, oncology, and fundamental neural science. We are scaling up our system for larger objects, such as the human breast and extremities, and are looking forward to making a profound contribution to clinical translation, especially for human breast cancer imaging.
Written by Lei Li, Liren Zhu and Lihong Wang.
Our paper: Li, L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 1, 0071 (2017).
References
1. Benveniste, H. & Blackband, S. MR microscopy and high resolution small animal MRI: applications in neuroscience research. Progress in neurobiology 67, 393-420 (2002).
2. Brenner, D.J. & Hall, E.J. Computed tomography—an increasing source of radiation exposure. New England Journal of Medicine 357, 2277-2284 (2007).
3. Greco, A. et al. Ultrasound biomicroscopy in small animal research: applications in molecular and preclinical imaging. BioMed Research International 2012 (2011).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in