The paper in Nature Biomedical Engineering is here: https://go.nature.com/2HYPyoh
The adult human heart cannot regenerate itself after injury. The death of cardiomyocytes irreversibly weakens the heart muscle until it can no longer pump blood adequately to supply the body. This is a challenge that our research group, along with the broader scientific community, seek to overcome in the treatment of heart disease.
The discovery of human pluripotent stem cells in the early 2000's caused a paradigm shift as it has been thought that transplantation of stem cells could lead to cardiomyocyte replacement and recovery of heart function. Armed with this new tool, the field started to develop strategies to generate adult cardiomyocytes and functional cardiac tissues for heart repair. While significant progress has been made in cell therapies of the heart, there was always a concern that the implantation of spontaneously contracting cardiomyocytes may lead to unwanted and potentially deadly arrhythmias. In fact, such events have been reported in large animal studies. As we watched the cells spontaneously contracting in culture over the course of our experiments in the lab (Video 1), we kept wondering how else we could apply these human cardiomyocytes therapeutically.

We were fortunate to work in the interdisciplinary lab of Dr. Gordana Vunjak-Novakovic. Dr. Aranazu Villasante, a post-doctoral researcher in the lab, had been studying tumor-secreted extracellular vesicles (EVs); inspired by her work, and by the growing body of literature on the multiple effects of EVs, we set out to explore whether our stem-cell-derived cardiomyocytes produced EVs. Fortuitously, they produced a lot (Video 2).

As tissue engineers, our goal is not limited to generating de novo constructs to replace damaged tissue, but also to devise strategies to empower native cells, nature’s best engineers. Motivated by their potential ability to regenerate the native cardiac tissue, we tested our bounty of EVs in a pilot study of several rats undergoing myocardial infarction. The difference between EV-treated and untreated animals was remarkable.
What followed was a truly multidisciplinary effort involving the engineering, biology, and medicine to develop and characterize a treatment strategy using the EVs secreted by human cardiomyocytes. First, we needed to devise a robust method to deliver EVs in a way that would prevent them from being washed away. We leveraged our lab's expertise on biomaterials and hydrogels to encapsulate the vesicles in a collagen-based patch which allowed sustained release into the post-injury environment over the course of several weeks.
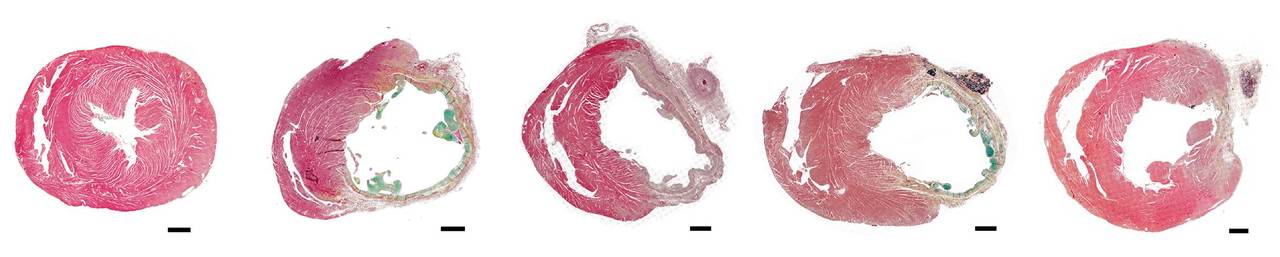
Throughout initial studies, we remained concerned about the possibility of arrhythmic complications, so we used continuous cardiac monitoring to track the electrical activity of treated rat hearts. We were pleased to see that EV-treated rats did not experience any more arrhythmias than untreated animals. Encouraged by these results, we moved on to see just how effective the EVs could be. With the help of Dr. Shunichi Homma, an expert in non-invasive cardiac imaging, we were able to see how much the cardiac function of these rats improved over the course of a month. Amazingly, when we analyzed the extent of the injury after a month, we found that cardiomyocyte-EV-treated rats had significantly reduced infarct sizes when compared with untreated controls.
At the same time, we were interested in understanding how our EVs were able achieve these striking effects. As previous studies of EVs from other cell types had demonstrated that EVs delivered microRNAs (miRNAs) capable of regulating molecular processes, we used next-generation sequencing to profile the miRNA content of our cardiomyocyte EVs with the help of Dr. Peter Sims, one of our close collaborators in systems biology. Remarkably, we found that cardiomyocyte EVs carried a variety of miRNAs, including many of those known to be active in modulating cardiac specific processes.
We believe that our work represents a step forward towards achieving the elusive goal of regenerating an injured heart. We see the cardiomyocytes derived from pluripotent stem cells as a powerful and untapped source of therapeutic EVs. EVs are easy to isolate and are stable when frozen over long periods of time, presenting an opportunity for off-the-shelf therapeutic products that can be used in acute injury settings, as opposed to cells which can take months to isolate and grow. We are now tasked with perhaps the more difficult questions of understanding how exactly these EVs are able to promote heart recovery. With such an understanding, we hope to help develop a safe and effective cell-free therapy for the infarcted heart.
Written by Bohao Liu & Benjamin Lee
Our paper: Liu, B. et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from iPS cells. Nat. Biomed. Eng. doi:10.1038/s41551-018-0229-7 (2018).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in