The discovery of Channelrhodopsins-1 and -2 (ChR)1,2 and the development of “Optogenetics” brought experimental manipulations in neuroscience to the next level in terms of temporal and spatial specificity. Since then neuroscientists have transferred ChR-2 and other microbial opsins into neurons to control neuronal activity with light and study the role of specific neuronal circuits in behavior. Although control of membrane depolarization using cation channelrhodopsins is well established, hyperpolarization still holds some limitations preventing its use in a variety of applications. Light-activated proton-, chloride-pumps and chloride-selective channelrhodopsins (ACRs) are the current standards used for inhibition. However, proton-pumps such as archeorhodopsin (Arch) can cause undesirable changes in cytoplasmic pH, and sustained activation at presynaptic terminal is associated with increased neurotransmitter release3. On the other hand, long lasting activation of ACR and Cl- pumps disturbs the chloride-electrochemical gradient at the plasma membrane and shifts chloride reversal potential to more positive values4. As a consequence chloride accumulation reduces the action potential threshold and changes cell excitability5,6. Additionally, activation of ACRs causes neuronal excitation instead of inhibition at high intracellular chloride concentrations, as is the case for neurons during development or in subcellular compartments such as the axons3,7. We were aware that those problems would fade away with a light-activated, well-expressed potassium channel. In our paper published in Nature Communications, we describe the development of a new potassium-based two-component optogenetic tool for silencing of excitable cells.
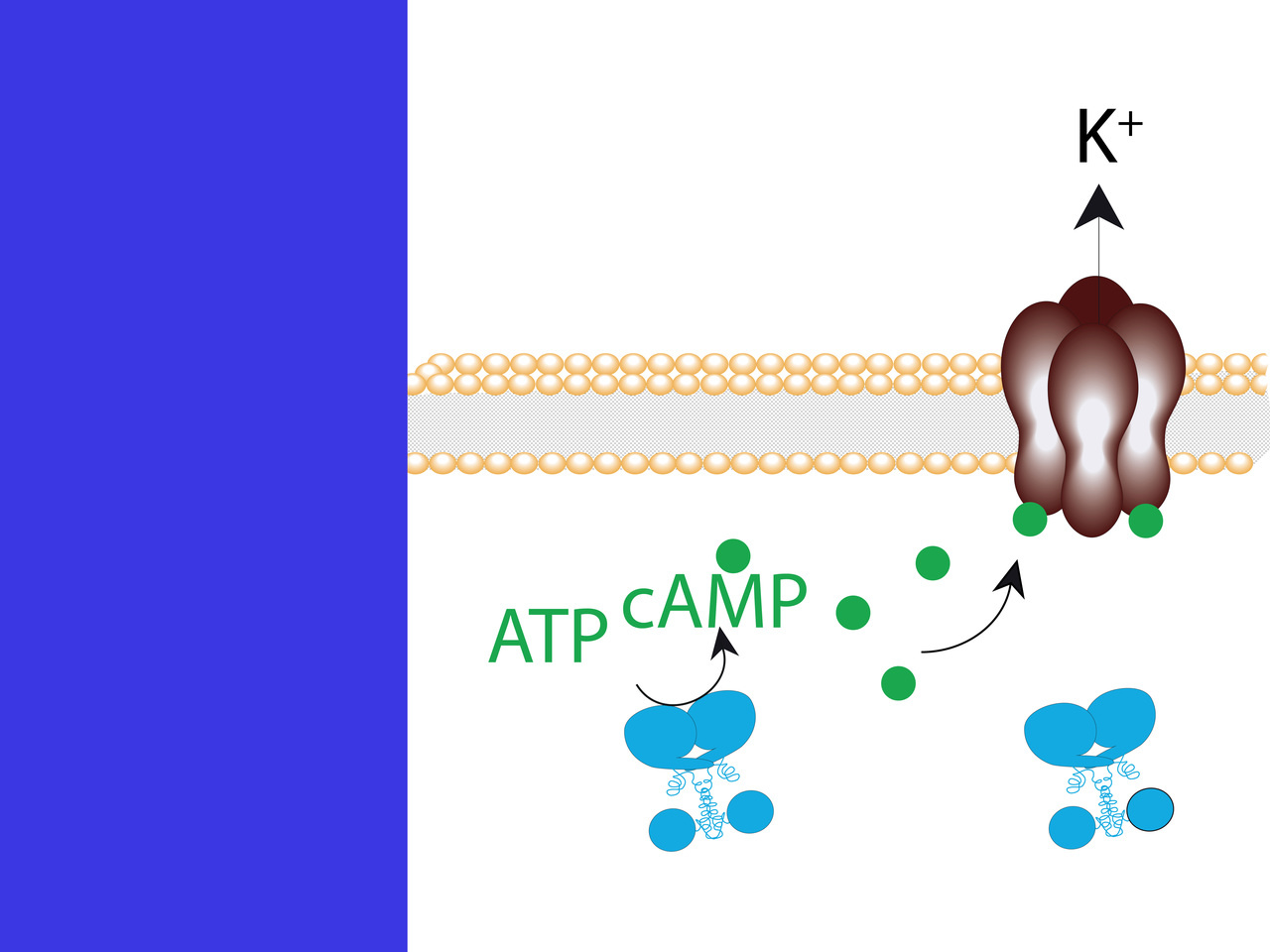
Everything started with the publication of a cAMP-gated potassium channel from the bacterium Spirochaeta thermophile (SthK)8. The authors could express and functionally characterize SthK in oocytes. For us it was a very good opportunity to combine this channel with our well-established light-activated adenylyl cyclase bPAC9 to develop an indirectly light-gated potassium channel. However, we envisioned two hurdles: First, we were skeptical about the effectiveness of the “PAC-K” system as inhibitory tool because bacterial channels are known for being poorly expressed in mammalian cells. Second, we did not know if the cAMP produced by bPAC would be sufficient to gate the channel considering the low open probability of SthK. We produced two different design-variants for the two-component system: a split version with SthK located at the membrane and bPAC in the cytoplasm (“split-PAC-K”), and a fused version (“fused-PAC-K”), in which the channel and the bPAC are covalently linked and are located at the plasma membrane (Fig-1a). Once expressed in cell lines both variants produced huge long-lasting outward potassium currents upon activation of bPAC with blue light (Fig-1b). The off-kinetics of the tool was considered as potential limitation, which is very slow when compared to channelrhodopsins. Fortunately, we soon realized that it is rather an advantage because due to the low-light intensity needed to activate the tool and the long lasting currents, PAC-K is the most sensitive inhibitory tool currently available.

Fig 1. Development of PAC-K as an inhibitory optogenetic tool. a, Molecular design of PAC-K. b, Representative current traces of PAC-K expressed in cell lines at different light-intensities. c, Inhibition of action potential in hippocampal slices of mouse brain.
We tested PAC-K function to inhibit neuronal activity in the nervous system of mice and zebrafish in vivo, and also in rabbit cardiomyocytes. In all these systems, PAC-K showed remarkably performance. Since optogenetics has also expanded to other research fields such as cellular signal transduction, cancer, immunology, pathogen-host interaction, among others; we are very optimistic that PAC-K will be a very useful tool for the scientific community.
Our paper: Bernal Sierra et al. Potassium channel-based optogenetic silencing. Nature Communications (2018).
References
1. Nagel, G. et al. Channelrhodopsin-1: A Light-Gated Proton Channel in Green Algae. Science 296, 2395–2398 (2002).
2. Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U. S. A. 100, 13940–13945 (2003).
3. Mahn, M., Prigge, M., Ron, S., Levy, R. & Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 (2016).
4. Wietek, J. Anion Conducting Channelrhodopsins. (Humboldt-Universität zu Berlin, 2018). doi:10.18452/19325
5. Sørensen, A. T. et al. Altered Chloride Homeostasis Decreases the Action Potential Threshold and Increases Hyperexcitability in Hippocampal Neurons. eNeuro 4, (2018).
6. Alfonsa, H. et al. The contribution of raised intraneuronal chloride to epileptic network activity. J. Neurosci. Off. J. Soc. Neurosci. 35, 7715–7726 (2015).
7. Gilbert, D. et al. Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int. J. Dev. Neurosci. 25, 479–489 (2007).
8. Brams, M., Kusch, J., Spurny, R., Benndorf, K. & Ulens, C. Family of prokaryote cyclic nucleotide-modulated ion channels. Proc. Natl. Acad. Sci. U. S. A. 111, 7855–7860 (2014).
9. Stierl, M. et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 286, 1181–1188 (2011).





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in