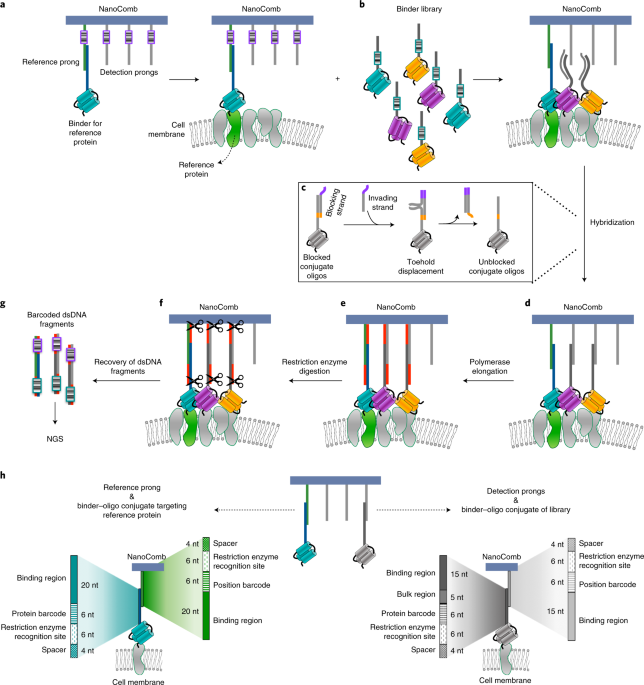
When you hear about DNA nanostructure maybe your mind goes directly to the images of very fancy and original shapes as result of a self-assembling process of specified DNA sequences. But beyond the creativity of researchers, since the development of the first DNA nanostructures by Ned Seeman, many DNA nanotechnology applications have been developed, moved by the fact that, as in most living organisms DNA functions as genetic information carrier, DNA assemblies could be used as a nanoscale tool to acquire and convey information. Inspired by that, we developed a DNA nanoassembly-based approach, termed NanoDeep, to decipher the nanoscale spatial distribution of membrane proteins. We exploited a simple DNA assembly, that we named NanoComb, to translate membrane protein organization information into a DNA sequencing readout, as shown in our article, just published on Nature Nanotechnology.

We demonstrated the application of NanoDeep to the analysis of protein nanoenvironments surrounding Her2, a membrane receptor of critical relevance in cancer. We showed that NanoDeep accommodates different types of binders, including affibodies, nanobodies, peptides and aptamers, which due to their small size do not limit the resolution of the method. The flexibility of NanoDeep regarding the types of binders facilitates further expansion of binder libraries. By applying NanoDeep to the analysis of the nanoenvironment surrounding the membrane protein Her2, we were able to simultaneously target six proteins.
The NanoDeep approach presented in this work has the potential to provide a breakthrough in the analysis of the spatial distribution of proteins in membrane nanoclusters without microscopy measurements, not only in cell cultures but eventually also in tissue samples. It overcomes the super-resolution imaging limitation on the number of proteins that can be analysed simultaneously and on its feasibility to image a small fraction of all protein nanodomains present in a cell population. Our method allows for the detection en masse of the inventory of proteins that forms the nanoenvironment of any reference membrane protein in cell populations.
As future perspective we will expand the library of binders by screening available small binders for their suitability for NanoDeep. The goal is to assemble a universal library with different types of binders that covers as much as possible the around 1500 membrane proteins in human cells. Our goal is that our method will shed light on the study of membrane protein organisation. Characterising the complexity and the differences in protein nanoenvironments in different cellular contexts has the potential to lead to the development of new therapeutic strategies aimed to target not the membrane receptors per se, but their spatial organisation.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in